LPUNEST syllabus – Lovely Professional University exam
LPUNEST syllabus – Lovely Professional University exam syllabus – LPUNEST is National Entrance and Scholarship Test for admission to various programmes in Engineering and technology in Lovely Professional University. After clearing this test and getting a good score, the applicant can hope to get a seat based on merit after studying basic 12 th standard board examination type questions.
Contents
- 1 LPUNEST syllabus – Lovely Professional University exam
- 2 CHEMISTRY
- 2.0.1 UNIT 1: Atomic Structure, States of Matter & Thermodynamics
- 2.0.2 UNIT 2: Solutions, Chemical Kinetics & Surface Chemistry
- 2.0.3 UNIT 3: Hydrogen & s – Block Element
- 2.0.4 UNIT 4: p, d & f block Elements and Environmental Chemistry
- 2.0.5 UNIT 5: Basic Concepts of Organic Chemistry
- 2.0.6 UNIT 6: Oxygen, Nitrogen, Polymers & Bio molecules
- 3 PHYSICS
- 4 MATHEMATICS
- 5 BIOLOGY
- 6 ENGLISH
LPUNEST syllabus – Lovely Professional University exam
CHEMISTRY
UNIT 1: Atomic Structure, States of Matter & Thermodynamics
Some basic concepts in chemistry: Matter and its nature, Dalton’s atomic theory; Concept of atom, molecule, element and compound; Physical quantities and their measurements in Chemistry, precision and accuracy, significant figures, S.I. Units, dimensional analysis; Laws of chemical combination; Atomic and molecular masses, mole concept, molar mass, percentage composition, empirical and molecular formulae; Chemical equations and stoichiometry.
States of matter: Classification of matter into solid, liquid and gaseous states.
Gaseous State: Measurable properties of gases; Gas laws – Boyle’s law, Charle’s law, Graham’s law of diffusion, Avogadro’s law, Dalton’s law of partial pressure; Concept of Absolute scale of temperature; Ideal gas equation; Kinetic theory of gases; Concept of average, root mean square and most probable velocities; Real gases, deviation from Ideal behaviour, compressibility factor and van der Waals equation, liquefaction of gases, critical constants.
Liquid State: Properties of liquids – vapour pressure, viscosity and surface tension and effect of temperature on them (qualitative treatment only).
Solid State: Classification of solids: molecular, ionic, covalent and metallic solids, amorphous and crystalline solids (elementary idea); Bragg’s Law and its applications; Unit cell and lattices, packing in solids (fcc, bcc and hcp lattices), voids, calculations involving unit cell parameters, imperfection in solids; Electrical, magnetic and dielectric properties.
Atomic structure: Discovery of sub-atomic particles (electron, proton and neutron), Thomson and Rutherford atomic models and their limitations; Nature of electromagnetic radiation, photoelectric effect; Spectrum of hydrogen atom, Bohr model of hydrogen atom – its postulates, derivation of the relations for energy of the electron and radii of the different orbits, limitations of Bohr’s model; Dual nature of matter, de-Broglie’s relationship, Heisenberg uncertainty principle. Elementary ideas of quantum mechanics, quantum mechanical model of atom, its important features, concept of atomic orbitals as one electron wave functions; Variation of ? and ?2 , with r for 1s and 2s orbitals; various quantum numbers (principal, angular momentum and magnetic quantum numbers) and their significance; shapes of s, p and d – orbitals, electron spin and spin quantum number; Rules for filling electrons in orbitals – aufbau principle, Pauli’s exclusion principle and Hund’s rule, electronic configuration of elements, extra stability of half- filled and completely filled orbitals.
Chemical bonding and molecular structure: Kossel – Lewis approach to chemical bond formation, concept of ionic and covalent bonds. Ionic Bonding: Formation of ionic bonds, factors affecting the formation of ionic bonds; calculation of lattice enthalpy.
Covalent Bonding: Concept of electronegativity, Fajan’s rule, dipole moment; Valence Shell Electron Pair Repulsion (VSEPR) theory and shapes of simple molecules.
Quantum mechanical approach to covalent bonding: Valence bond theory – Its important features, concept of hybridization involving s, p and d orbitals; Resonance.
Molecular Orbital Theory: Its important features, LCAOs, types of molecular orbitals (bonding, antibonding), sigma and pi-bonds, molecular orbital electronic configurations of homonuclear diatomic molecules, concept of bond order, bond length and bond energy. Elementary idea of metallic bonding. Hydrogen bonding and its applications.
Chemical thermodynamics:Fundamentals of thermodynamics: System and surroundings, extensive and intensive properties, state functions, types of processes.
First law of thermodynamics:Concept of work, heat internal energy and enthalpy, heat capacity, molar heat capacity; Hess’s law of constant heat summation; Enthalpies of bond dissociation, combustion, formation, atomization, sublimation, phase transition, hydration, ionization and solution.
Second law of thermodynamics: Spontaneity of processes; ?S of the universe and ?G of the system as criteria for spontaneity, ?G0(Standard Gibbs energy change) and equilibrium constant.
UNIT 2: Solutions, Chemical Kinetics & Surface Chemistry
Solutions: Different methods for expressing concentration of solution – molality, molarity, mole fraction, percentage (by volume and mass both), vapour pressure of solutions and Raoult’s Law – Ideal and non-ideal solutions, vapour pressure – composition, plots for ideal and non-ideal solutions; Colligative properties of dilute solutions – relative lowering of vapour pressure, depression of freezing point, elevation of boiling point and osmotic pressure; Determination of molecular mass using colligative properties; Abnormal value of molar mass, van’t Hoff factor and its significance.
Equilibrium: Meaning of equilibrium, concept of dynamic equilibrium.
Equilibria involving physical processes: Solid -liquid, liquid – gas and solid – gas equilibria, Henry’s law, general characteristics of equilibrium involving physical processes.
Equilibria involving chemical processes: Law of chemical equilibrium, equilibrium constants (Kp and Kc) and their significance, significance of ?G and ?Go in chemical equilibria, factors affecting equilibrium concentration, pressure, temperature, effect of catalyst; Le Chatelier’s principle.
Ionic equilibrium: Weak and strong electrolytes, ionization of electrolytes, various concepts of acids and bases (Arrhenius, Bronsted – Lowry and Lewis) and their ionization, acid – base equilibria (including multistage ionization) and ionization constants, ionization of water, pH scale, common ion effect, hydrolysis of salts and pH of their solutions, solubility of sparingly soluble salts and solubility products, buffer solutions.
Redox reactions and Electrochemistry: Electronic concepts of oxidation and reduction, redox reactions, oxidation number, rules for assigning oxidation number, balancing of redox reactions. Electrolytic and metallic conduction, conductance in electrolytic solutions, specific and molar conductivities and their variation with concentration: Kohlrausch’s law and its applications.
Electrochemical cells – Electrolytic and Galvanic cells, different types of electrodes, electrode potentials including standard electrode potential, half – cell and cell reactions, emf of a Galvanic cell and its measurement; Nernst equation and its applications; Relationship between cell potential and Gibbs’ energy change; Dry cell and lead accumulator; Fuel cells; Corroison and its prevention.
Chemical Kinetics: Rate of a chemical reaction, factors affecting the rate of reactions: concentration, temperature, pressure and catalyst; elementary and complex reactions, order and molecularity of reactions, rate law, rate constant and its units, differential and integral forms of zero and first order reactions, their characteristics and half – lives, effect of temperature on rate of reactions – Arrhenius theory, activation energy and its calculation, collision theory of bimolecular gaseous reactions (no derivation).
Surface chemistry: Adsorption- Physisorption and chemisorption and their characteristics, factors affecting adsorption of gases on solids – Freundlich and Langmuir adsorption isotherms, adsorption from solutions. Catalysis- Homogeneous and heterogeneous, activity and selectivity of solid catalysts, enzyme catalysis and its mechanism.
Colloidal State – distinction among true solutions, colloids and suspensions, classification of colloids-lyophilic, lyophobic; multi molecular, macromolecular and associated colloids (micelles), preparation and properties of colloids – Tyndall effect, Brownian movement, electrophoresis, dialysis, coagulation and ?occulation; Emulsions and their characteristics.
UNIT 3: Hydrogen & s – Block Element
Classi?cation of elements and periodicity in properties: Modern periodic law and present form of the periodic table, s, p, d and f block elements, periodic trends in properties of elements atomic and ionic radii, ionization enthalpy, electron gain enthalpy, valence, oxidation states and chemical reactivity.
General principles and processes of isolation of metals: Modes of occurrence of elements in nature, minerals, ores; Steps involved in the extraction of metals – concentration, reduction (chemical and electrolytic methods) and refining with special reference to the extraction of Al, Cu, Zn & Fe; Thermodynamics and electrochemical principles involved in the extraction of metals.
Hydrogen: Position of hydrogen in periodic table, isotopes, preparation, properties & uses of hydrogen; Physical & Chemical properties of water & Heavy Water; Structure, preparation, reactions & uses of hydrogen peroxide; Classification of hydrides – ionic, covalent and interstitial, Hydrogen as a fuel.
s – Block elements (alkali and alkaline earth metals) Group – 1 and 2 Elements: General introduction, electronic configuration and general trends in physical and chemical properties of elements, anomalous properties of the first element of each group, diagonal relationships. Preparation and properties of some important compounds – sodium carbonate, sodium chloride, sodium hydroxide and sodium hydrogen carbonate; Industrial uses of lime, limestone, Plaster of Paris and cement; Biological significance of Na, K, Mg and Ca.
UNIT 4: p, d & f block Elements and Environmental Chemistry
p – Block elements
Group – 13 to Group – 18 Elements: General Introduction: Electronic configuration and general trends in physical and chemical properties of elements across the periods and down the groups; unique behaviour of the first element in each group. GroupWise study of the p – block elements.
Group – 13: Preparation, properties and uses of boron and aluminium; structure, properties and uses of borax, boric acid, diborane, boron tri- ?uoride, aluminium chloride and alums.
Group – 14: Tendency for catenation; Structure, properties & uses of allotropes and oxides of carbon, silicon tetrachloride, silicates, zeolites and silicones.
Group – 15: Properties & uses of nitrogen and phosphorus; Allotrophic forms of phosphorus; Preparation, properties, structures and uses of ammonia, nitric acid, phosphine and phosphorus halides, (PCl3, PCl5); Structures of oxides and oxoacids of nitrogen and phosphorus.
Group – 16: Preparation, properties, structures & uses of dioxygen and ozone; Allotropic forms of sulphur; Preparations, properties, structures & uses of sulphur dioxide, sulphuric acid (including its industrial preparation); Structures of oxoacids of sulphur.
Group – 17: Preparation, properties and uses of hydrochloric acid; Trends in the acidic nature of hydrogen halides; Structures of interhalogen compounds and oxides & oxoacids of halogens.
Group – 18: Occurrence and uses of noble gases; Structures of ?uorides and oxides of xenon.
d – and f – Block elements: Transition Elements: General introduction, electronic configuration, occurrence and characteristics, general trends in properties of the first row transition elements – physical properties, ionization enthalpy, oxidation states, atomic radii, colour, catalytic behaviour, magnetic properties, complex formation, interstitial compounds, alloy formation; Preparation, properties and uses of K2 Cr2 O7 and KMnO4 .
Inner Transition Elements: Lanthanoids – Electronic configuration, oxidation states, chemical reactivity and lanthanoid contraction. Actinoids – Electronic configuration and oxidation states.
Co-ordination Compounds: Introduction to Co-ordination compounds, Werner’s theory; ligands, co-ordination number, denticity, chelation; IUPAC nomenclature of mononuclear co- ordination compounds, isomerism; Bonding-Valence bond approach and basic ideas of Crystal field theory, colour and magnetic properties; Importance of co-ordination compounds (in qualitative analysis, extraction of metals and in biological systems).
Environmental chemistry: Environmental pollution- Atmospheric, water and soil.
Tropospheric pollutants – Gaseous pollutants: Oxides of carbon, nitrogen and sulphur, hydrocarbons; their sources, harmful effects and prevention; Green house effect and Global warming; Acid rain; Particulate pollutants: Smoke, dust, smog, fumes, mist; their sources, harmful effects and prevention.
Stratospheric pollution: Formation and breakdown of ozone, depletion of ozone layer -its mechanism and effects. Water Pollution – Major pollutants such as, pathogens, organic wastes and chemical pollutants; their harmful effects and prevention. Soil pollution – Major pollutants such as: Pesticides (insecticides, Herbicides and fungicides), their harmful effects and prevention. Strategies to control environmental pollution.
UNIT 5: Basic Concepts of Organic Chemistry
Puri?cation and characterization of organic compounds: Puri?cation: Crystallization, sublimation, distillation, differential extraction and chromatography – principles and their applications.
Qualitative analysis: Detection of nitrogen, sulphur, phosphorus and halogens.
Quantitative analysis (Basic Principles only): Estimation of carbon, hydrogen, nitrogen,halogens, sulphur, phosphorus. Calculations of empirical formulae and molecular formulae; Numerical problems in organic quantitative analysis.
Some basic principles of organic chemistry: Tetravalency of carbon; Shapes of simple molecules – hybridization (s and p); Classification of organic compounds based on functional groups: – C = C – , – C ? C – and those containing halogens, oxygen, nitrogen and sulphur; Homologous series; Isomerism – structural and stereoisomerism.
Nomenclature (Trivial and IUPAC) Covalent bond ?ssion: Homolytic and heterolytic: free radicals, carbocations and carbanions; stability of carbocations and free radicals, electrophiles and nucleophiles.
Electronic displacement in a covalent bond – Inductive effect, electromeric effect, resonance and hyperconjugation. Hydrocarbons: Classification, isomerism, IUPAC nomenclature, general methods of preparation, properties & reactions.
Common types of organic reactions– Substitution, addition, elimination and rearrangement.
Alkanes – Conformations: Sawhorse and Newman projections (of ethane); Mechanism of halogenations of Alkanes.
Alkenes – Geometrical isomerism; Mechanism of electrophilic addition: addition of hydrogen, halogens, water, hydrogen halides (Markownikoff’s and peroxide effect); Ozonolysis, oxidation and polymerization.
Alkynes – Acidic character; Addition of hydrogen, halogens, water and hydrogen halides; Polymerization.
Aromatic hydrocarbons – Nomenclature, benzene – structure and aromaticity; Mechanism of electrophilic substitution: halogenations, nitration, Friedel – Craft’s alkylation and acylation, directive in?uence of functional group in mono- substituted benzene.
Organic compounds containing halogens: General methods of preparation, properties and reactions; Nature of C-X bond; Mechanisms of substitution reactions. Uses; Environmental effects of chloroform & iodoform, freons and DDT.
UNIT 6: Oxygen, Nitrogen, Polymers & Bio molecules
Organic compounds containing Oxygen: General methods of preparation, properties, reactions and uses.
Alcohols: Identification of primary, secondary and tertiary alcohols; mechanism of dehydration.
Phenols: Acidic nature, electrophilic substitution reactions: halogenations, nitration and sulphonation, Reimer – Tiemann reaction.
Ethers: Structure.
Aldehyde and Ketones: Nature of carbonyl group; Nucleophilic addition to >C=O group, relative reactivities of aldehydes and ketones; Important reactions such as – Nucleophilic addition reactions (addition of HCN, NH3 and its derivatives), Grignard reagent; oxidation; reduction (Wolff Kishner and Clemmensen); acidity of – hydrogen, aldol condensation, Cannizzaro reaction, Haloform reaction; Chemical tests to distinguish between aldehydes and Ketones.
Carboxylic acids: Acidic strength and factors affecting it.
Organic compounds containing Nitrogen: General methods of preparation, properties, reactions and uses.
Amines: Nomenclature, classification, structure, basic character and identification of primary, secondary and tertiary amines and their basic character.
Diazonium Salts: Importance in synthetic organic chemistry.
Polymers: General introduction and classification of polymers, general methods of polymerization-addition and condensation, copolymerization; Natural and synthetic rubber and vulcanization; some important polymers with emphasis on their monomers and uses – polythene, nylon, polyester and bakelite.
Biomolecules: General introduction and importance of biomolecules.
Carbohydrates: Classification: aldoses and ketoses; monosaccharides (glucose and fructose) and constituent monosaccharides of oligosacchorides (sucrose, lactose and maltose) and polysaccharides (starch, cellulose, glycogen).
Proteins: Elementary Idea of amino acids, peptide bond, polypeptides; Proteins: primary, secondary, tertiary and quaternary structure (qualitative idea only), denaturation of proteins, enzymes.
Vitamins: Classification and functions.
Nucleic acids: Chemical constitution of DNA and RNA. Biological functions of nucleic acids.
Chemistry in everyday life: Chemicals in medicines– Analgesics, tranquilizers, antiseptics, disinfectants, antimicrobials, antifertility drugs, antibiotics, antacids antihistamines-their meaning & common example.
Chemicals in food – Preservatives, artificial sweetening agents – common examples.
Cleansing agents – Soaps and detergents, cleansing action.
PHYSICS
UNIT 1: Motion
Laws of motion: Force and Inertia, Newton’s First Law of motion; Momentum, Newton’s Second Law of motion; Impulse; Newton’s Third Law of motion. Law of conservation of linear momentum and its applications; equilibrium of concurrent forces. Static and Kinetic friction, laws of friction, rolling friction. Dynamics of uniform circular motion: Centripetal force and its applications.
Work, energy and power: Work done by a constant force and a variable force; kinetic and potential energies, work energy theorem, power. Potential energy of a spring, conservation of mechanical energy, conservative and non-conservative forces; Elastic & inelastic collisions in one and two dimensions.
Rotational motion: Centre of mass of a two-particle system, Centre of mass of a rigid body; Basic concepts of rotational motion; moment of a force, torque, angular momentum, conservation of angular momentum and its applications; moment of inertia, radius of gyration. Values of moments of inertia for simple geometrical objects, parallel and perpendicular axes theorems and their applications. Rigid body rotation, equations of rotational motion.
UNIT 2: Kinematics, Gravitation & Oscillations
Physics and measurement: Physics, technology and society, SI units, Fundamental and derived units. Least count, accuracy and precision of measuring instruments, Errors in measurement, Dimensions of Physical quantities, dimensional analysis and its applications.
Kinematics: Frame of reference. Motion in a straight line: Position-time graph, speed and velocity. Uniform and non- uniform motion, average speed and instantaneous velocity, uniformly accelerated motion, velocity- time, position-time graphs, relations for uniformly accelerated motion. Scalars and Vectors, Vector addition and Subtraction, Zero Vector, Scalar and Vector products, Unit Vector, Resolution of a Vector. Relative Velocity, Motion in a plane, Projectile Motion, Uniform Circular Motion.
Gravitation: The universal law of gravitation. Acceleration due to gravity and its variation with altitude and depth. Kepler’s laws of planetary motion. Gravitational potential energy; gravitational potential. Escape velocity. Orbital velocity of a satellite. Geo-stationary satellites.
Oscillations and waves:Periodic motion – period, frequency, displacement as a function of time. Periodic functions. Simple harmonic motion (S.H.M.) and its equation; phase; Oscillations of a spring – restoring force and force constant; energy in S.H.M. : kinetic and potential energies; Simple pendulum – derivation of expression for its time period; Free, forced and damped oscillations, resonance; Wave motion; Longitudinal and transverse waves, speed of a wave; Displacement relation for a progressive wave; Principle of superposition of waves, re?ection of waves Standing waves in strings and organ pipes, fundamental mode and harmonics, Beats, Doppler effect in sound.
UNIT 3: Thermal Physics
Properties of solids and liquids: Elastic behaviour, Stress- strain relationship, Hooke’s Law, Young’s modulus, Bulk modulus, modulus of rigidity. Pressure due to a ?uid column; Pascal’s law and its applications. Viscosity, Stokes’ law, terminal velocity, streamline and turbulent ?ow, Reynolds number. Bernoulli’s principle and its applications. Surface energy and surface tension, angle of contact, application of surface tension – drops, bubbles and capillary rise. Heat, temperature, thermal expansion; specific heat capacity, calorimetry; change of state, latent heat. Heat transfer conduction, convection and radiation, Newton’s law of cooling.
Thermodynamics: Thermal equilibrium, zeroth law of thermodynamics, concept of temperature. Heat, work and internal energy. First law of thermodynamics. Second law of thermodynamics: reversible and irreversible processes. Carnot engine and its efficiency.
Kinetic theory of gases:Equation of state of a perfect gas, work done on compressing a gas. Kinetic theory of gases – assumptions, concept of pressure. Kinetic energy and temperature: rms speed of gas molecules; Degrees of freedom, Law of equipartition of energy, applications to specific heat capacities of gases; Mean free path, Avogadro’s number.
UNIT 4: Electricity & Magnetism
Electrostatics: Electric charges: Conservation of charge, Coulomb’s law-forces between two point charges, forces between multiple charges; superposition principle and continuous charge distribution. Electric field: Electric field due to a point charge, Electric field lines, Electric dipole, Electric field due to a dipole, Torque on a dipole in a uniform electric field. Electric ?ux, Gauss’s law and its applications to find field due to infinitely long uniformly charged straight wire, uniformly charged infinite plane sheet and uniformly charged thin spherical shell. Electric potential and its calculation for a point charge, electric dipole and system of charges; Equipotential surfaces, Electrical potential energy of a system of two point charges in an electrostatic field. Conductors and insulators, Dielectrics and electric polarization, capacitor, combination of capacitors in series and in parallel, capacitance of a parallel plate capacitor with and without dielectric medium between the plates, Energy stored in a capacitor.
Current electricity: Electric current, Drift velocity, Ohm’s law, Electrical resistance, Resistances of different materials, V-I characteristics of Ohmic and nonohmic conductors, Electrical energy and power, Electrical resistivity, Colour code for resistors; Series and parallel combinations of resistors; Temperature dependence of resistance. Electric Cell and its internal resistance, potential difference and emf of a cell, combination of cells in series and in parallel. Kirchhoff’s laws and their applications. Wheatstone bridge, Metre bridge. Potentiometer – principle and its applications.
Magnetic effects of current and magnetism: Biot – Savart law and its application to current carrying circular loop. Ampere’s law and its applications to infinitely long current carrying straight wire and solenoid. Force on a moving charge in uniform magnetic and electric fields. Cyclotron. Force on a current-carrying conductor in a uniform magnetic field. Force between two parallel current-carrying conductors- definition of ampere. Torque experienced by a current loop in uniform magnetic field; Moving coil galvanometer, its current sensitivity and conversion to ammeter and voltmeter. Current loop as a magnetic dipole and its magnetic dipole moment. Bar magnet as an equivalent solenoid, magnetic field lines; Earth’s magnetic field and magnetic elements. Para-, dia- and ferro- magnetic substances. Magnetic susceptibility and permeability, Hysteresis, Electromagnets and permanent magnets.
UNIT 5: Atomic Structure & Optics
Atoms and nuclei: Alpha-particle scattering experiment; Rutherford’s model of atom; Bohr model, energy levels, hydrogen spectrum. Composition and size of nucleus, atomic masses, isotopes, isobars; isotones. Radioactivity-alpha, beta and gamma particles/rays and their properties; radioactive decay law. Mass-energy relation, mass defect; binding energy per nucleon and its variation with mass number, nuclear fission and fusion.
Dual nature of matter and radiation: Dual nature of radiation. Photoelectric effect, Hertz and Lenard’s observations; Einstein’s photoelectric equation; particle nature of light. Matter waves-wave nature of particle, de Broglie relation. Davisson-Germer experiment.
Optics:Re?ection and refraction of light at plane and spherical surfaces, mirror formula, Total internal re?ection and its applications, Deviation and Dispersion of light by a prism, Lens Formula, Magnification, Power of a Lens, Combination of thin lenses in contact, Microscope and Astronomical Telescope (re?ecting and refracting) and their magnifying powers.
Wave optics:wavefront and Huygens’ principle, Laws of re?ection and refraction using Huygen’s principle. Interference, Young’s double slit experiment and expression for fringe width. Diffraction due to a single slit, width of central maximum. Resolving power of microscopes and astronomical telescopes, Polarisation, plane polarized light; Brewster’s law, uses of plane polarized light and Polaroids.
UNIT 6: Electrical & Electronics
Electromagnetic induction and alternating currents: Electromagnetic induction; Faraday’s law, induced emf and current; Lenz’s Law, Eddy currents. Self and mutual inductance. Alternating currents, peak and rms value of alternating current/ voltage; reactance and impedance; LCR series circuit, resonance; Quality factor, power in AC circuits, wattless current. AC generator and transformer.
Electromagnetic waves:Electromagnetic waves and their characteristics. Transverse nature of electromagnetic waves. Electromagnetic spectrum (radio waves, microwaves, infrared, visible, ultraviolet, X- rays, gamma rays). Applications of e.m. waves.
Electronic devices: Semiconductors; semiconductor diode: I-V characteristics in forward and reverse bias; diode as a rectifier; I-V characteristics of LED, photodiode, solar cell and Zener diode; Zener diode as a voltage regulator. Junction transistor, transistor action, characteristics of a transistor; transistor as an amplifier (common emitter configuration) and oscillator. Logic gates (OR, AND, NOT, NAND and NOR). Transistor as a switch. Communication systems: Propagation of electromagnetic waves in the atmosphere; Sky and space wave propagation, Need for modulation, Amplitude and Frequency Modulation, Bandwidth of signals, Bandwidth of Transmission medium, Basic Elements of a Communication System.
MATHEMATICS
UNIT 1: Algebra
Sets, relations and functions: Sets and their representation; Union, intersection and complement of sets and their algebraic properties; Power set; Relation, Types of relations, equivalence relations; Functions, oneone, into and onto functions, composition of functions.
Complex numbers and quadratic equations: Complex numbers as ordered pairs of reals, Representation of complex numbers in the form a+ib and their representation in a plane, Argand diagram, algebra of complex numbers, modulus and argument (or amplitude) of a complex number, square root of a complex number, triangle inequality, Quadratic equations in real and complex number system and their solutions. Relation between roots and coefficients, nature of roots, formation of quadratic equations with given roots.
Sequences and series:Arithmetic and Geometric progressions, insertion of arithmetic, geometric means between two given numbers. Relation between A.M. and G.M. Sum upto n terms of special series: Geometric progression.
UNIT 2: Matrices, Vectors & Reasoning
Matrices & Determinants: Matrices, algebra of matrices, types of matrices, determinants and matrices of order two and three, Adjoint, transpose, symmetric and skew symmetric matrices, Properties of determinants, evaluation of determinants, area of triangles using determinants. evaluation of inverse of a square matrix using determinants and elementary transformations, Test of consistency and solution of simultaneous linear equations in two or three variables using determinants and matrices.
Vector algebra: Vectors and scalars, addition of vectors, components of a vector in two dimensions and three dimensional space, scalar and vector products, scalar and vector triple product.
Mathematical reasoning: Statements, logical operations and, or, implies, implied by, if and only if. Understanding of tautology, contradiction, converse and contrapositive.
UNIT 3: Permutation-Combinations & Binomial Theorem
Permutations and Combinations: Fundamental principle of counting, permutation as an arrangement and combination as selection, Meaning of P (n, r) and C (n, r), simple applications.
Mathematical induction:Principle of Mathematical Induction and its simple applications.
Binomial theorem and its simple applications: Binomial theorem for a positive integral index, general term and middle term, properties of Binomial coefficients and simple applications.
UNIT 4: Limit, Integration & Differentiation
Limit, continuity and differentiability: Real valued functions, algebra of functions, polynomials, rational, trigonometric, logarithmic and exponential functions, inverse functions. Graphs of simple functions. Limits, continuity and differentiability. Differentiation of the sum, difference, product and quotient of two functions. Differentiation of trigonometric, inverse trigonometric, logarithmic, exponential, composite and implicit functions; derivatives of order upto two. Rolle’s and Lagrange’s Mean Value Theorems. Applications of derivatives: Rate of change of quantities, monotonic increasing and decreasing functions, Maxima and minima of functions of one variable, tangents and normals.
Integral calculus: Integral as an anti derivative. Fundamental integrals involving algebraic, trigonometric, exponential and logarithmic functions. Integration by substitution, by parts and by partial fractions. Integration using trigonometric identities. Evaluation of simple integrals of the type:
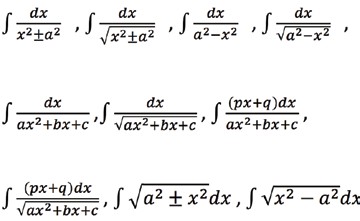
Integral as limit of a sum. Fundamental Theorem of Calculus. Properties of definite integrals. Evaluation of definite integrals, determining areas of the regions bounded by simple curves in standard form. Differential equations: Ordinary differential equations, their order and degree. Formation of differential equations. Solution of differential equations by the method of separation of variables, solution of homogeneous and linear differential equations of the type:
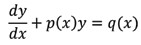
UNIT 5: Geometry
Coordinate geometry: Cartesian system of rectangular coordinates in a plane, distance formula, section formula, locus and its equation, translation of axes, slope of a line, parallel and perpendicular lines, intercepts of a line on the coordinate axes.
Straight lines: Various forms of equations of a line, intersection of lines, angles between two lines, conditions for concurrence of three lines, distance of a point from a line, equations of internal and external bisectors of angles between two lines, coordinates of centroid, orthocentre and circumcentre of a triangle, equation of family of lines passing through the point of intersection of two lines.
Circles, conic sections:Standard form of equation of a circle, general form of the equation of a circle, its radius and centre, equation of a circle when the end points of a diameter are given, points of intersection of a line and a circle with the centre at the origin and condition for a line to be tangent to a circle, equation of the tangent. Sections of cones, equations of conic sections (parabola, ellipse and hyperbola) in standard forms, condition for y = mx + c to be a tangent and point (s) of tangency.
Three Dimensional Geometry: Coordinates of a point in space, distance between two points, section formula, direction ratios and direction cosines, angle between two intersecting lines. Skew lines, the shortest distance between them and its equation. Equations of a line and a plane in different forms, intersection of a line and a plane, coplanar lines.
UNIT 6: Probability & Trigonometry
Statistics and probability: Measures of Dispersion: Calculation of mean, median, mode of grouped and ungrouped data calculation of standard deviation, variance and mean deviation for grouped and ungrouped data.
Probability: Probability of an event, addition and multiplication theorems of probability, Baye’s theorem, probability distribution of a random variate, Bernoulli trials and Binomial distribution.
Trigonometry: Trigonometrical Identities and equations, Trigonometrical functions, Inverse trigonometric function: Definition, domain, range, elementary properties of inverse trignometric functions, Heights and Distances.
BIOLOGY
Unit 1: Diversity & Structural Organisation
What is living? Biodiversity; Need for classification; Three domains of life; Taxonomy & Systematics; Concept of species and taxonomical hierarchy; Binomial nomenclature; Tools for study of Taxonomy – Museums, Zoos, Herbaria, Botanical gardens.
Five kingdom classi?cation: Salient features and classification of Monera; Protista and Fungi into major groups; Lichens; Viruses and Viroids, Salient features and classification of plants into major groups-Algae, Bryophytes, Pteridophytes, Gymnosperms and Angiosperms; Angiosperms- classification up to class, characteristic features and examples, Salient features and classification of animals-nonchordate up to phyla level and chordate up to classes level.
Structural Organisation in Animals and Plants: Morphology and modifications; Tissues; Anatomy and functions of different parts of ?owering plants: Root, stem, leaf, in?orescence- cymose and recemose, ?ower, fruit and see, Animal tissues; Morphology, anatomy and functions of different systems (digestive, circulatory, respiratory, nervous and reproductive) of an insect (cockroach).
Cell Structure and Function : Cell theory and cell as the basic Unit of life; Structure of prokaryotic and eukaryotic cell; Plant cell and animal cell; Cell envelope, cell membrane, cell wall; Cell organelles-structure and function; Endomembrane system-endoplasmic reticulum, Golgi bodies, lysosomes, vacuoles; mitochondria, ribosomes, plastids, micro bodies; Cytoskeleton, cilia, ?agella, centrioles (ultra structure and function); Nucleus-nuclear membrane, chromatin, nucleolus. Chemical constituents of living cells: Biomolecules-structure and function of proteins, carbodydrates, lipids, nucleic acids; Enzymes-types, properties, enzyme action;
Cell division: Cell cycle, mitosis, meiosis and their significance.
Unit 2: Plant Physiology
Transport in plants: Movement of water, gases and nutrients; Cell to cell transport-Diffusion, facilitated diffusion, active transport; Plant – water relations – Imbibition, water potential, osmosis, plasmolysis; Long distance transport of water – Absorption, apoplast, symplast, transpiration pull, root pressure and guttation; Transpiration-Opening and closing of stomata; Uptake and translocation of mineral nutrients- Transport of food, Phloem transport, Mass ?ow hypothesis; Diffusion of gases.
Mineral nutrition: Essential minerals, macro and micronutrients and their role; Deficiency symptoms; Mineral toxicity; Elementary idea of Hydroponics as a method to study mineral nutrition; Nitrogen metabolism-Nitrogen cycle, Biological Nitrogen fixation.
Photosynthesis: Photosynthesis as a means of Autotrophic nutrition; Site of photosynthesis take place; pigments involved in Photosynthesis (Elementary idea); Photochemical and biosynthetic phases of photosynthesis; Cyclic and non cyclic and photophosphorylation; Chemiosmotic hypothesis; Photorespiration C3 and C4 pathways; Factors affecting photosynthesis.
Respiration: Exchange gases; Cellular respiration- glycolysis, fermentation (anaerobic), TCA cycle and electron transport system (aerobic); Energy relations- Number of ATP molecules generated; Amphibolic pathways; Respiratory quotient.
Plant growth and development: Seed germination; Phases of Plant growth and plant growth rate; Conditions of growth; Plant growth and development: Seed germination; Conditions of growth; Differentiation, dedifferentiation and redifferentiation; Sequence of developmental process in a plant cell; Growth regulators-auxin, gibberellin, cytokinin, ethylene, ABA; Seed dormancy; Vernalisation; Photoperiodism.
Unit 3: Human Physiology
Digestion and absorption: Alimentary canal and digestive glands; Role of digestive enzymes and gastrointestinal hormones; Peristalsis, digestion, absorption and assimilation of proteins, carbohydrates and fats; Caloric value of proteins, carbohydrates and fats; Egestion; Nutritional and digestive disorders – PEM, indigestion, constipation, vomiting, jaundice, diarrhea.
Breathing and Respiration: Respiratory organs in animals; Respiratory system in humans; Mechanism of breathing and its regulation in humans- Exchange of gases, transport of gases and regulation of respiration Respiratory volumes; Disorders related to respiration-Asthma, Emphysema, Occupational respiratory disorders.
Body ?uids and circulation: Composition of blood, blood groups, coagulation of blood; Composition of lymph and its function; Human circulatory system-Structure of human heart and blood vessels; Cardiac cycle, cardiac output, ECG, Double circulation; Regulation of cardiac activity; Disorders of circulatory system-Hypertension, Coronary artery disease, Angina pectoris, Heart failure.
Excretory products and their elimination: Modes of excretion- Ammonotelism, ureotelism, uricotelism; Human excretory system-structure and function; Urine formation, Osmoregulation; Regulation of kidney function-Renin- angiotensin, Atrial Natriuretic Factor, ADH and Diabetes insipidus; Role of other organs in excretion; Disorders; Uraemia, Renal failure, Renal calculi, Nephritis; Dialysis and artificial kidney.
Locomotion and Movement: Types of movement- ciliary, fiagellar, muscular; Skeletal muscle- contractile proteins and muscle contraction; Skeletal system and its functions; Joints; Disorders of muscular and skeletal system-Myasthenia gravis, Tetany, Muscular dystrophy, Arthritis, Osteoporosis, Gout.
Neural control and coordination: Neuron and nerves; Nervous system in humans- central nervous system, peripheral nervous system and visceral nervous system; Generation and conduction of nerve impulse; Re?ex action; Sense organs; Elementary structure and function of eye and ear.
Chemical coordination and regulation: Endocrine glands and hormones; Human endocrine system-Hypothalamus, Pituitary, Pineal, Thyroid, Parathyroid, Adrenal, Pancreas, Gonads; Mechanism of hormone action; Role of hormones as messengers and regulators, Hypo-and hyperactivity and related disorders (Common disorders e.g. Dwarfism, Acromegaly, Cretinism, goiter, exopthalmicgoiter, diabetes, Addison’s disease).
Unit 4: Reproduction, Genetics and Evolution
Reproduction in organisms: Reproduction, a characteristic feature of all organisms for continuation of species; Modes of reproduction – Asexual and sexual; Asexual reproduction; Modes-Binary fission, sporulation, budding, gemmule, fragmentation; vegetative propagation in plants.
Sexual reproduction in ?owering plants: Flower structure; Development of male and female gametophytes; Pollination- types, agencies and examples; Outbreeding devices; Pollen- Pistil interaction; Double fertilization; Post fertilization events- Development of endosperm and embryo, Development of seed and formation of fruit; Special modes-apomixis, parthenocarpy, polyembryony; Significance of seed and fruit formation.
Human Reproduction: Male and female reproductive systems; Microscopic anatomy of testis and ovary; Gametogenesis- spermatogenesis & oogenesis; Menstrual cycle; Fertilisation, embryo development upto blastocyst formation, implantation; Pregnancy and placenta formation; Parturition; Lactation.
Reproductive health: Need for reproductive health and prevention of sexually transmitted diseases (STD); Birth control-Need and Methods, Contraception and Medical Termination of Pregnancy (MTP); Amniocentesis; Infertility and assisted reproductive technologies – IVF, ZIFT, GIFT.
Genetics and Evolution:Heredity and variation: Mendelian Inheritance; Deviations from Mendelism- Incomplete dominance, Co-dominance, Multiple alleles and Inheritance of blood groups, Pleiotropy; Elementary idea of polygenic inheritance; Chromosome theory of inheritance; Chromosomes and genes; Sex determination-In humans, birds, honey bee; Linkage and crossing over; Sex linked inheritance-Haemophilia, Colour blindness; Mendelian disorders in humans-Thalassemia; Chromosomal disorders in humans; Down’s syndrome, Turner’s and Klinefelter’s syndromes.
Molecular basis of Inheritance: Search for genetic material and DNA as genetic material; Structure of DNA and RNA; DNA packaging; DNA replication; Central dogma; Transcription, genetic code, translation; Gene expression and regulation- Lac Operon; Genome and human genome project; DNA finger printing.
Evolution: Origin of life; Biological evolution and evidences for biological evolution from Paleontology, comparative anatomy, embryology and molecular evidence; Darwin’s contribution, Modern Synthetic theory of Evolution; Mechanism of evolution-Variation (Mutation and Recombination) and Natural Selection with examples, types of natural selection; Gene ?ow and genetic drift; Hardy-Weinberg’s principle; Adaptive Radiation; Human evolution.
Unit 5: Biology, Biotechnology and Human Welfare
Health and Disease: Pathogens; parasites causing human diseases (Malaria, Filariasis, Ascariasis Typhoid, Pneumonia, common cold, amoebiasis, ring worm); Basic concepts of immunology-vaccines; Cancer, HIV and AIDS; Adolescence, drug and alcohol abuse.
Improvement in food production: Plant breeding, tissue culture, single cell protein, Biofortification; Apiculture and Animal husbandry.
Microbes in human welfare: In household food processing, industrial production, sewage treatment, energy generation and as biocontrol agents and biofertilizers.
Biotechnology and Its Applications: Principles and process of Biotechnology: Genetic engineering (Recombinant DNA technology).
Application of Biotechnology in health and agriculture: Human insulin and vaccine production, gene therapy; Genetically modified organisms-Bt crops; Transgenic Animals; Biosafety issues-Biopiracy and patents.
Unit 6: Ecology and environment
Organisms and environment: Habitat and niche; Population and ecological adaptations; Population interactions- mutualism, competition, predation, parasitism; Population attributes-growth, birth rate and death rate, age distribution.
Ecosystem: Patterns, components; productivity and decomposition; Energy ?ow; Pyramids of number, biomass, energy; Nutrient cycling (carbon and phosphorous); Ecological succession; Ecological Services-Carbon fixation, pollination, oxygen release.
Biodiversity and its conservation: Concept of Biodiversity; Patterns of Biodiversity; Importance of Biodiversity; Loss of Biodiversity; Biodiversity conservation; Hotspots, endangered organisms, extinction, Red Data Book, biosphere reserves, National parks and sanctuaries.
Environmental issues: Air pollution and its control; Water pollution and its control; Agrochemicals and their effects; Solid waste management; Radioactive waste management; Greenhouse effect and global warning; Ozone depletion; Deforestation; Any three case studies as success stories addressing environmental issues.
ENGLISH
Grammar:Parts of speech – Noun, Pronoun, Adjective, Adverb, Verb, Preposition, Conjunction Interjection; Tenses – Present, Past and Future Tense in Active and Passive Form; Modal Verbs – Can, Could, May, might, Should, Will, Would.
Associative Language Skills: Vocabulary – Antonyms, Synonyms, One word substitution, Word Analogies, Idioms and Phrases
Common Errors: Sentence Correction and Error Finding Exercises
Comprehension Passages :Closed and Open paragraphs, identifying key ideas or theme.